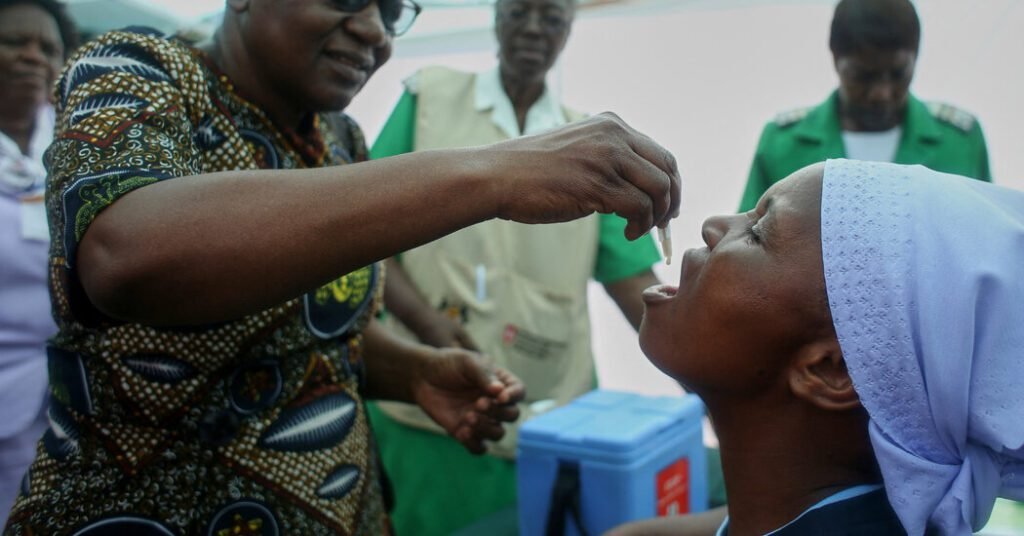
Cholera vaccine doses are being given to patients as fast as they are produced, and the global supply has been completely depleted as deadly cases of the disease continue to spread.
This comes as no shock to anyone in the field of emergency response because vaccine stocks have been precariously low for years.
The surprise — the good news, which is surprising in itself, since “cholera” and “good news” are rarely used together — is that three new vaccine makers are setting up production lines and joining the effort to replenish the stockpile.
And a fourth company, the only one currently making the vaccine, which is given by mouth, is working at what experts describe as a “heroic” pace to expand production.
However, even with all this, the total global supply of vaccine available this year will be, at best, a quarter of what is needed.
As of late February, countries had already reported 79,300 cases and 1,100 deaths from cholera this year. Since there is no uniform system for counting cases, this is likely a gross underestimate.
In October 2022, the agency that manages the global emergency cholera vaccine stockpile made an unprecedented recommendation that people receive only one dose of the vaccine instead of two in an effort to expand the supply. A single dose of the cholera vaccine provides six months to two years of immunity, while the full regimen of two doses given one month apart gives adults about four years of protection.
Last year, countries sent requests for 76 million doses of the vaccine for one-dose “reactive campaigns” — efforts to vaccinate people in places with active outbreaks.
There were only 38 million doses in stock, so only half the requests were met, and those were only with a single dose. No vaccines remained for preventive campaigns that would ideally take place in places like Gaza, where all the conditions for large outbreaks exist, or in places where cholera is endemic.
The race to produce more cholera vaccine shows all the reasons why it is so difficult to respond to outbreaks, even with the involvement of dedicated drugmakers who are not afraid of the small profit margins on a vaccination aimed mostly at poor people.
Cholera can cause death from dehydration in as little as a day, as the body tries to expel infectious bacteria in streams of vomit and watery diarrhea. The disease is transmitted through unclean drinking water. The current outbreaks are due to the spread of conflict and climate disasters that force people into crowded living conditions without adequate sanitation systems. In recent months, there have been outbreaks in 17 countries, including Afghanistan, Zambia and Syria.
However, demand has only grown since then.
The South Korean company EuBiologics is currently the only company in the world that manufactures the cholera vaccine. The company has known for some time that there would be pressure to supply the vaccine because the only other company that made it, an Indian subsidiary of pharmaceutical company Sanofi, had announced in 2018 that it would end production of the vaccine, which made the 2023.
To fill the gap in vaccine production, Rachel Park, director of international operations at EuBiologics, said the company decided to try to simplify the vaccine formula, modernizing steps and ingredients so it could make more doses faster.
The company was then making more of the bulk drug than it could quickly put into tubes, so it agreed with a second Korean company to help.
EuBiologics also invested in building a second production facility that would double the amount of vaccine the company could make. The company has taken the long and expensive steps to get both the simplified vaccine and its new facility approved by the World Health Organization in a process called pre-selection, which means countries won’t have to manage their own regulatory reviews. . When the new plant starts production, the company will be able to produce up to 46 million doses per year.
“EuBiologics is really the unsung hero of the story,” said Dr. Julia Lynch, director of the cholera vaccine program for the International Vaccine Institute, a UN-backed agency based in Seoul. “They are doing everything they can to get the volumes up as quickly as possible.”
Together, these steps will increase production in total to about 46 million doses this year and to about 90 million doses in 2025 and beyond, Ms. Park said. But this will probably be much less than what the world demands.
“The doses are distributed before they are even produced,” said Dr. Daniela Garone, the international medical coordinator for Doctors Without Borders who sits on the committee that decides which countries will receive doses and how many. “We didn’t expect it to be any better this year, but we didn’t think it would be this bad.”
There’s more hope on the horizon: Three more pharmaceutical companies have cholera vaccines in the pipeline. The International Vaccine Institute has licensed his vaccine to Biological E, an Indian company, and shares the formula and equipment to make it. WHO
In South Africa, a company called Biovac will soon begin clinical trials for what could ultimately be the first vaccine ever produced from start to finish in sub-Saharan Africa. Biovac hopes to complete trials by 2027. After that, it will likely take at least a year to get the vaccine the WHO’s default, said Dr. Morena Makhoana, Biovac’s chief executive.
Bharat Biotech, another large Indian company with large production capacity, is working on its own oral cholera vaccine. It could release its vaccine by the end of 2025.
To encourage companies to invest in cholera vaccine production, Gavi, the international agency that supplies vaccinations to low- and middle-income countries, has suggested the possibility of advance market commitments — the promise of future orders that would encourage pharmacists to invest in the production of the cholera vaccine. Gavi pays EuBiologics $1.53 per dose for the vaccine.
Bharat and Biological E plan to initially produce about 15 million doses a year, said Dr. Lynch — “moderate volumes” by the standards of these huge Indian companies that could do more if the market continues to grow.
Potential demand is difficult to predict, he said. “That’s really the question: Is what the world is going through right now some kind of a few-year phenomenon that’s triggered by something?” said Dr. Lynch. “Or is this a new normal? Is this a new kind of set point?’