The Federal Trade Commission and the Department of Health and Human Services said Wednesday they will look into the causes of generic drug shortages and the practices of “powerful middlemen” involved in the supply chain.
The federal agency investigation is targeting syndicates and drug distributors that have come under fire in recent months as drug shortages hit a 10-year high. The agencies want to examine the companies’ influence on how the drugs are sold to hospitals and other health facilities, assessing whether middlemen exerted pressure on pricing and manufacturing that led to harm.
During congressional hearings last year, oncology experts testified about the impact of the shortages, describing difficult decisions that forced them to procure essential chemotherapy drugs. They described month-to-month, sometimes week-to-week, gaps in supplies that posed deadly risks to some patients.
“For years Americans have faced acute shortages of critical drugs, from chemotherapy to antibiotics, putting patients at risk,” said Lina Khan, the chair of the FTC. “Our research seeks insight into the factors driving these shortages and scrutinizes the practices of opaque drug intermediaries.”
In earlier interviews with The Times, generic drug industry executives had expressed deeper concerns about their reliance on three major group purchasing organizations for contracts to sell drugs to hospital and health center clients. Generic executives complained that their companies sometimes offered below-market prices to win big contracts, a strategy that had eroded stability in the industry, especially among makers of sterile injectables often used in surgery and cancer treatment.
Lawmakers echoed the concerns. Late last year, Sen. Ron Wyden, D-Oregon and chairman of the Senate Finance Committee, criticized “very powerful healthcare middlemen” in the generic drug industry. Last month, he and Sen. Mike Crapo, R-Idaho, outlined ways to curb drug shortages, focusing in part on proposed changes to Medicare payments for sterile injectable drugs.
Dr. Robert Califf, the commissioner of the Food and Drug Administration, testified to Congress last year about the limits on the agency’s ability to manage drug shortages, pointing to market dynamics — such as low and falling prices — in the generic drug industry.
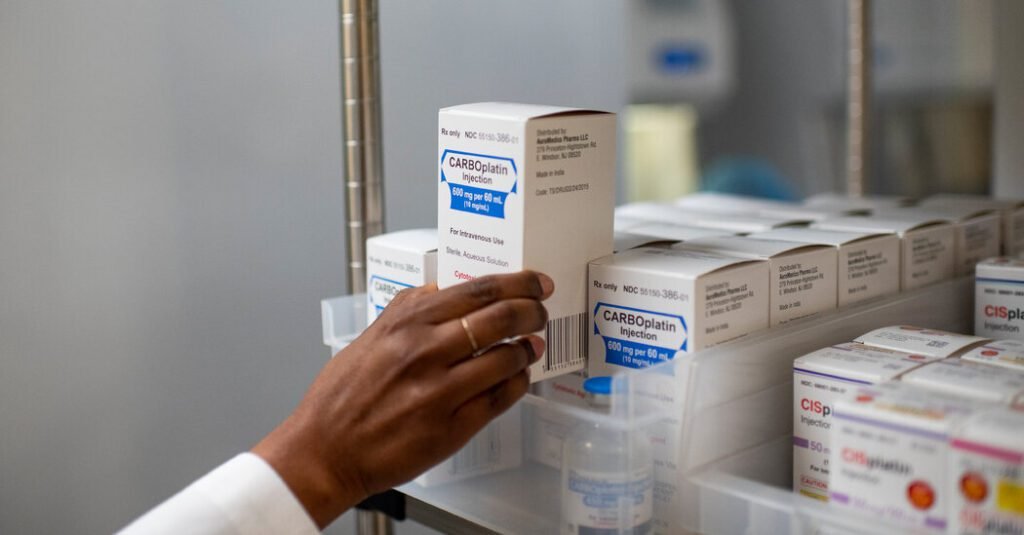
Shortages of chemotherapy drugs have made headlines for lawmakers and the drug industry. Cancer experts were forced to draw up treatment guidelines that recommended giving rare doses to those patients who had a chance to cure—and denying them to patients with metastatic disease who wanted to live longer.
The key chemotherapy drugs that were in short supply, cisplatin and carboplatin, are vital for the treatment of lung, breast, testicular, ovarian and head and neck cancers. In recent years, prices of both drugs have fallen to about $15 to $20 per dose, even as Intas Pharmaceuticals, an India-based drug company, has gained market share.
Intas halted production of the drugs amid quality concerns stemming from a surprise FDA inspection in late 2022. That resulted in wider shortages, which generic drug industry executives pointed to as an example of how which falling prices and take-all contracts have increased reliance on fewer drug makers.
The FTC’s investigation announced Wednesday focuses on whether concentration among drug industry intermediaries “has discouraged suppliers from competing in generic drug markets.” The agency is accepting public comments as part of its investigation into the deficiencies.
The Association for Accessible Medicines, a trade group for the generic drug industry, praised the FTC for trying to address the issue. David Gaugh, the group’s interim president, said in a statement that it was important for the agency to look at lower generic drug prices, consolidation among intermediary companies and reductions in manufacturing facilities.
“As a result of all this, the risk of drug shortages will only increase without action to strengthen the long-term sustainability of generic production,” Mr Gaugh said in a statement.
The federal investigation is expected to examine the three main group-buying organizations that deal with generic drug manufacturers to supply drugs to hundreds of customers. Todd Ebert, president of the Healthcare Supply Chain Association, which represents group buyers, said the companies provide competitive prices to hospitals and other health care providers — as well as a reliable supply of drugs.
“GPOs help stabilize the generic drug market by partnering with manufacturers in contracts that provide the certainty and predictable demand they need to stay on the market,” Mr. Ebert said in a statement. He added that the agency looks forward to sharing more with the FTC about the critical role GPOs play in addressing the ongoing drug shortage crisis.
The Healthcare Distributors Alliance, which represents major companies such as McKesson, Cardinal Health and AmerisourceBergen that assess fees to generic drugmakers to carry their drugs, also did not respond to requests for comment.